February 12th, 2015 §
 Once again, it’s been a while since I’ve updated. Somehow most of you know that with me usually no news does not actually equal good news. Before I give an update I want to remind readers that I do update the blog Facebook page (click here to reach it) with short medical updates more often these days if you are interested.
Once again, it’s been a while since I’ve updated. Somehow most of you know that with me usually no news does not actually equal good news. Before I give an update I want to remind readers that I do update the blog Facebook page (click here to reach it) with short medical updates more often these days if you are interested.
Most of the last three weeks since I last wrote have been dealing still with swelling and blood count issues from radiation and from the extensive disease in my liver that we are working on with the radiation and with chemo. I’ve needed numerous transfusions of red cells for anemia and quite a few for platelets which have really come under attack. We have been taking a break from the Epirubicin and I got a dose of Faslodex (I had it last year as well in a clinical trial with a trial drug, this month I am using it with a daily pill, Letrozole). Rather than being cytotoxic chemos (traditional “cell-killers”) these are anti hormonal agents. We are just trying to let my body recover now for a few weeks.
Last week my abdomen grew and grew with fluid from the liver and overall inflammation. By the end of the week I had an abdomen the size I was when I was ready to give birth. The skin was painfully stretched. We needed to intervene. I went to urgent care for evaluation for a paracentesis (draining/”tap” of fluid). My platelets were too low. They transfused and I just made the cutoff. The process is pretty simple: visualize if the pocket of fluid is drainable (tiny pockets would not be) via ultrasound.
Three needles of increasing gauge are inserted to numb through to the pocket. A catheter is inserted and attached to a drainage bottle that has a bit of vacuum to draw out the fluid. Two and one half liters later we were done. It is a huge amount to see. I did have soreness at the site and discomfort from such a lot of fluid removal after. But this is a very common procedure with metastases to the liver (please, please remember: breast cancer that metastasizes to the liver is not liver cancer. Mets to the brain are not brain cancer. They are breast cancer cells that have moved in the body. There can be/are big differences in terms of prognosis, treatment, chemotherapy agents, etc).
Some patients only need a few of these abdominal taps. Some need them every few weeks depending on how chemo, etc. are working. Sometimes a drain is actually placed to allow a constant ability to remove fluid.
If you were following me last year you know I had a drain placed in my lung while in the hospital that accomplished something similar. I was fortunate to only need that one for a few weeks (I had already had a one-time tap on my left lung called a thoracentesis). For now there are no plans for a drain or other more serious interventions in my abdomen. Now that my potassium levels are holding steady we are using diuretics to manage the issue.
We are working on the plan for next week, it is likely that I will be going back to a chemotherapy called Xeloda (these are pills taken daily) for a bit because it is gentler on blood counts. I’ll update when we have a decision on that.
I’m weak, still unable to drive, or do much more than walk than to the bathroom or a car that is picking me up. I’ve had to adjust some things at home including bathroom rails and so on. Safety is most important and especially with very low platelets falls and any bleeding or injury risk is a serious concern.
I’m sure I’ve forgotten something (or things) but I do want to get this out today. I see snow out the window but I’m still focused on Spring. These hydrangeas in my room this week reminded me March is not far off.
Thank you to those near and far for all different kinds of support given this month with an extra shout-out to my wonderful team at Sloan-Kettering. If any of my doctors, nurses, or support staff there are reading this: you help make these tough days better. I value your care always.
December 18th, 2013 §
 “This one has to work,” she says, “It just has to.”
“This one has to work,” she says, “It just has to.”
These are the words my phlebotomist says to me every time I see her. She says it strongly, willing it to be so.
I wish it were that easy. If wanting it could make it so… all of the people who send their support in prayers, thoughts, hopes, vibes, whatever it is they hope will help… all of those would work. And yet, here we are. Through no fault of theirs, or mine.
It is important to see the larger picture here, aside from my own life. It is important for everyone reading this blogpost to know that despite all of the hype and exclamation points and strong language about a cure or the promise of successful long-term targeted agents for metastatic breast cancer so that it can be more like a “chronic disease,” we are not there yet. The number who can live like that are the minority. Most live in this life and death game of Whack-a-Mole that I do now: metastases (“mets”) pop up, and you try to bash them back down but as you do they pop up somewhere else.
The state of metastatic breast cancer care is that you can’t just test your breast cancer, look on a chart to find the drug that will work and always shut it down. Metastatic breast cancer has eluded this formula so far. We don’t have drugs yet to even target every mutation. And we don’t know which inhibitors work. Most work best in combination with other treatments and we have to have clinical trials to test all of those options. All of those things take something those of us living with MBC don’t necessarily have: the luxury of time. In my case, I have a type of mutation called Pi3k-alpha in my cancer. I took an investigational drug that was a Pi3k-alpha inhibitor (along with another drug). On paper it should have worked. It was the most advanced type of targeted treatment I could get for that mutation.
Cancer is complicated. It has multiple pathways to get fuel. Block one? It finds another. And even when you have a drug that shows results in mice or in a few other people, you don’t know if it will work for you. There are too many variables, too many drivers of cancer in complicated feedback loops.
You can see where this is going. I have come out on the wrong end of the equation yet again. The trial drug combination did not work.
I’m no longer continuing on the clinical trial of GDC-0032 and Faslodex that I’ve been on for 8 weeks. The trial is required to drop me and we (my team and I) agree it’s not wise to stay anyway.
My CT scan showed stability in my disease in the bones, lymph nodes and lung nodules. That’s good.
But we have other more serious concerns now. The breathing problems I was having we knew were due to a pleural effusion which initially worsened 6-8 weeks ago and then seemed to improve about a month ago. I have still been aware of breathing issues throughout the day but it hasn’t had too much impact on daily functioning so I have just pushed through.
We now have confirmation that the pleural effusion is larger than in the last scan. The fluid that is causing the trouble is from metastases to the pleura (not to be confused with metastases to the actual lungs, the pleura is the sac that the lungs sit in. They usually have a trace amount of fluid present. This amount is a lot more. The fluid associated with the cancer has settled in the left lower lobe and has displaced the lung upward). In plain terms, there is cancer in the pleura that is producing fluid that builds up in that normally thin sac beyond what can be drained by the normal body process.
Additionally, my liver is now affected as well, unfortunately. There are mulitiple lesions that are metastases as well. This is obviously something I was hoping to avoid for a while longer.
The nature of metastatic breast cancer is that you don’t know how fast things will move or where the cancer cells will settle and thrive. They like the environment of soft tissues (liver, brain, etc.) so these developments are not surprising nor what I want to be hearing.
We need to get aggressive in a new way now. Anti-hormonal agents and inhibitors have not been working for me even though on paper they “should.” Treatments that logically should work might not. And that’s why I get angry when some very visible people in breast cancer care want to keep talking about how “close” we are to personalized treatments and even cures. The research has yet to support that idea. In fact, the latest research has repeatedly shown how complex the interactions are. We now know there are more than 30 subtypes of breast cancer. And even those subtypes don’t always respond alike to treatments.
Cancer is wily. And I hope I’m wrong about how far away we are from true leaps and bounds in MBC care. But I know I won’t see it in my lifetime. For how many decades now have we been hearing about those “breakthroughs” and “miracle drugs”? Yes, they’ve come in some cancers. But not MBC. Reporters and health care professionals in the public eye need to monitor how they spin info about the current state of metastatic breast cancer treatments. Let’s not send the message out about how “close” we are to a cure when there isn’t research to back it up. Let’s not send a falsely reassuring message out there that metastatic breast cancer doesn’t need much attention because soon we’ll be able to make it like a chronic disease anyway. Until we have actually done that, we must push full steam ahead and not encourage complacency in research.
Stepping off my soapbox to come back down to my life, what does that mean for me now? We must choose a new game plan. The one we talked about only days ago doesn’t seem the best option anymore (that’s one reason I don’t take the time to go into my plan B here when it’s still a hypothetical. You always have to be ready to adjust based on new information). We’re huddling and tossing around some options. I should know by Friday when I go back to my last clinic appointment on the trial. In all likelihood I’ll be going to traditional chemotherapy.
For now, it’s hard news to hear, especially at the holidays. I was originally diagnosed with early breast cancer on December 20, 2006. That anniversary approaches. I search for the beauty each day. I make myself find it. I won’t give up these days even when they are so hard. Today as I drove the kids to school the full moon sat above the horizon. It was beautiful in the blue sky after our gray day of snow and rain yesterday. We all looked at it. And I was glad to be able to see it with them.
As many of you already know, my first tweet of each day is a mantra I’ve written: “Find a bit of beauty in the world today. Share it. If you can’t find it, create it. Some days this may be hard to do. Persevere.” I love to start my day with that saying each morning. It centers me. And so many have responded that they like it too.
This news is not good. But as always, I go forward. As I’ve written elsewhere:
Cellular biology is King,
But paired to that fateful ruler I shall be a rebellious, argumentative Queen.
……………………..
One side note: with the popularity of my Twitter feed and the New York Times feature, my volume of email has soared in the past few months. I get so much mail, often with long stories and also requests for advice and guidance about cancer treatment and coping. I am so sorry to say that I cannot always respond to these letters. I hope everyone understands this. I am flattered but the time it takes to fully respond would be like writing a blogpost to each person. If the requests are easy, I try to answer as many as I can. I read every email that comes in and every comment on the blogposts. Any answers to emails come only from me. So I just am hoping that everyone understands that during these turbulent times, I won’t be able to reply to individual requests for advice and care. Thank you for understanding.
November 14th, 2013 §
 Tuesday was a full day for me at Memorial Sloan-Kettering for Cycle 1, Day 16 of my current clinical trial.
Tuesday was a full day for me at Memorial Sloan-Kettering for Cycle 1, Day 16 of my current clinical trial.
With snow flakes and rain mixing in the morning rush, I decided to skip the train and go early with my husband as he made his usual morning commute to New York City by car. I knew this would get me to the office quite early. I was the first to arrive in the waiting room and didn’t mind the long wait. It gets very busy and noisy later in the day and I’m always happy to sit in warmth and comfort of a quiet room. I arrived at about 7:20 and my first appointment wasn’t scheduled until 8:30. I was fasting, so I did miss my morning coffee quite a bit while waiting, though, especially as other patients began to arrive and use the coffee machine!
I meet with the research nurse first. She weighs me (down 2 pounds from two weeks ago), takes my blood pressure (oops, very high using the machine, we’ll retake it manually, oh that’s much better, thank you very much), pulse (in the 90s), oxygen saturation (better than last time, now normal).
We review how I am feeling on the Faslodex and GDC-0032 combination. This is the first time I am discussing it with the team since starting the protocol. She goes through a list of symptoms and asks about each one. The list ranges from gastrointestinal issues (very important to monitor on this investigational drug) to neuropathy to dizziness to fatigue to rash. I am pleased to report that I feel quite good on the combo. I experienced a few days of hot flashes and night sweats from the first injections of Faslodex two weeks ago, I tell her, but that was it. From the GDC I really only have had appetite loss, metallic taste in my mouth, a bit of muscle pain, and slight fatigue as the most noticeable issues. In general, we both agree, I am tolerating it very well.
The biggest change I’ve experienced in the last two weeks is a drastic change in my breathing– for the better. I’m quite sure, I tell her, that my pleural effusion (excess fluid in the lining around the lungs) is almost resolved. This is great news and I am anxious to find out if my doctor hears the change. I also tell her that after much thought I have decided to have a port placed to make my blood draws and tracer injections for CT and bone scans easier.
Next I meet with my own oncologist. Usually I would meet with the Principal Investigator for this appointment. She was not in the office on the usual days this week, though, and I must have my check-ins on certain days in the cycle. I was told about the switch in schedules well in advance. The plan for the long term anyway is that I will transition back to my own oncologist for monitoring. The two doctors work closely together and there is no problem in doing this.
I haven’t seen my oncologist in what seems like a while (probably about one month) and we are happy to be “reunited” for this appointment. We review how I am doing clinically. She also gives me a physical exam and listens to my lungs. Yes, she agrees, there is a big change for the better. We discuss the port, and she orders the needed additional bloodwork for it.
We talk about the trial and other topics. As always, she asks about my family and writing. I tell her about the song I wrote and promise to send her a link to it. I tell her I have been invited to come talk to the class of Fellows at Sloan-Kettering in a few weeks about caring for the metastatic breast cancer patient. I am truly excited about this invitation. There isn’t anything I can think of that is more important than talking to young physicians about ways to make doctors and patients partners in care in light of a metastatic cancer diagnosis. I’ll share more about that visit as the time approaches.
Next I do scheduling for the next appointment in 2 weeks. That will be a much shorter day than today. Each appointment in the first 8 weeks on the protocol has different elements. Today will be a long day. Next time will just be a visit with the Principal Investigator and a blood draw.
 Next I head upstairs to the chemo suite where they have a room waiting for me. They have already called downstairs while I am doing scheduling to tell me which room to go to so I don’t even need to check in. The nurse I had last time comes in to the chemo room. I immediately tell her that she did a wonderful job with the two huge injections of Faslodex last time. I tell her the slow injection rate seems to have worked; I was sore for days (expected) but no bruising or welts. I always try to give as many compliments as I can; I think these oncology jobs must be very stressful and I would bet the complaints come often. If possible, I try to find something good and comment on it. Everyone likes being appreciated.
Next I head upstairs to the chemo suite where they have a room waiting for me. They have already called downstairs while I am doing scheduling to tell me which room to go to so I don’t even need to check in. The nurse I had last time comes in to the chemo room. I immediately tell her that she did a wonderful job with the two huge injections of Faslodex last time. I tell her the slow injection rate seems to have worked; I was sore for days (expected) but no bruising or welts. I always try to give as many compliments as I can; I think these oncology jobs must be very stressful and I would bet the complaints come often. If possible, I try to find something good and comment on it. Everyone likes being appreciated.
Today I will need to have my blood drawn, take my GDC-0032 pills, wait four hours, and have my blood drawn again. I must always wait one hour after taking the pills to eat. By the time I do all of this today it will be noon. The nurse gets a vein in my wrist easily for the required 12 vials and when we see it’s a good flow we agree to leave the line in and try to use it four hours from now and avoid another stick. I’m reactive to adhesive so she wraps me up carefully and I slide my sweater sleeve back down.
I take my pills with a full glass of water and document the time in the medication diary that I am required to keep. Then it’s time to drop my trousers for the two big Faslodex injections. I am not too nervous about this part now that I’ve done it once already. I will get these today, again in two weeks, and then monthly thereafter. I have mastered the art of muscle relaxation for these shots and use a trick of putting all of my weight on one foot while they inject the opposite side. Again the shots are uncomfortable because the viscous liquid is getting rammed into a muscle. But she does it slowly again as she is supposed to, and we’re done quickly. I still am self-conscious as the lightly-frosted sliding glass doors are all that stands between my tush and the hallway, but I’m already over that. I think the idea of facing the wall and clutching a table as needles are jabbed in my backside is probably more laughable.
I put on my coat, knowing the worst is over for the day and go meet a friend for lunch after waiting the required time to eat.
Later, I return directly to the same room I was previously in at the appointed time. The line still in my wrist yields a good flow, a few more tubes are drawn, and we’re done for the day.
Seven hours after first entering the building I am free to go. I still have two more weeks’ worth of the GDC-0032 in my bag and so I do not need to visit the pharmacy. At the next visit my medication log will be checked and my remaining pills will be counted and confiscated before I am given another 30 day supply.
…….
Over the last two days I have ended up needing two more blood draws, met with my local oncologist, and got my monthly Xgeva shot. After 7 needles in 3 days I’m glad to now have a few days off. I ended up needing to repeat one of the tests required for the port placement procedure (Tests related to clotting are routine before any surgical procedure. One of these was slightly elevated and I needed to repeat it. The second test came back just fine).
I also had my tumor markers done. This blood test is not a part of the clinical trial requirements. I’ve decided to watch these to see what they do while taking the drug. I’m sure some people would not choose to do this. This week’s test showed the numbers were quite elevated over previous weeks. We don’t know what that means. I have no increased pain, none of my other bloodwork shows any reason for concern. The numbers sometimes rise on new therapy before they drop. My breathing is significantly improved. So, we focus on the clinical signs and will just have to see what the markers do. I wish they had dropped, but I’ve seen the imperfect nature of this test time and again. I am able to realize this is only one piece of the puzzle right now. It doesn’t affect anything about my treatment plan. A reminder: in this trial success is measured by doing CT scans at varying intervals. My first one will be five weeks from now, at the seven week mark. Only then is a determination made about whether it is working, which would be disease stability or reduction. If the overall “quantity” of cancer is seen as growing by 20% or more, I would discontinue the trial and need to move to something else; it would be considered ineffective for me.
I was sore far more quickly after the Faslodex injections this time even though they weren’t any more painful at administration time. By the time I was on the train back home in the late afternoon I was hurting. For three days now I’ve had a heating pad on as much as possible. It just feels good. The seat heater in my car is getting a workout too. I know it will only be another day or two so I am not too bothered.
I don’t yet have a date scheduled for the port placement. That could be as soon as next week. I also have meetings with my cardiologist (these inhibitors can drastically change lipid levels. My cholesterol and triglycerides on my previous drug, Afinitor, went quite high. Thankfully they are coming down rapidly now but we still need to watch them carefully.) and my endocrinologist (my thyroid levels are now abnormal again. I have Hashimoto’s Disease which is usually very easy to manage but chemo does sometimes cause changes). I’m not in a huge rush to get the port, I just would like to have it in by the time I need to do the next round of scans.
I’m glad to finally be done for the week with appointments, hard to believe the weekend is almost here. I will see what next week brings and then it will be time to be back at MSK on the 25th of November for Cycle 2, day 1.
October 30th, 2013 §
 There is so much to say about the start of the phase 2 clinical trial earlier this week. I think it’s important to be as complete as I can on the main parts. I really want readers to get a sense of what it’s like to go through this and also what to expect if they decide to enter a trial themselves.
There is so much to say about the start of the phase 2 clinical trial earlier this week. I think it’s important to be as complete as I can on the main parts. I really want readers to get a sense of what it’s like to go through this and also what to expect if they decide to enter a trial themselves.
That said, it is obvious to me (and hopefully to you) that my experience is very unique. I have no earthly clue what other trials are like. I just want to make all of the disclaimers that I think you all know already: this is my experience only. If that helps in some way, great. But it can’t possibly tell you what another clinical trial might be like.
I still think it’s valuable. And I know I would have wanted to read posts like these a few weeks ago when I was signing up for the trial. So that’s my guide: if I think it would have helped me, I’m going to share it.
Twitter friends have been telling me that they are interested in five main topics: 1) what is the science behind this drug? 2) what does the treatment consist of/logistics 3) how did I choose this trial out of the ones available 4) side effects physically 5) effects emotionally. The last two will obviously be the ones we follow over time. I won’t be able to address all of those topics here but I’m getting a good jump on them.
This post is long. I’ve opted to just publish it and not divide it up. If you want to read it in chunks you can decide how to divide it up. If you want to skim the science parts, you’ll still have my report of my day at the end. I look forward to hearing your questions and comments. If you have questions I will try to answer them.
First, a bit about the drugs and the science behind them. My trial has me taking 6 mg of an investigational drug called GDC-0032 made by Genentech every morning. I receive two injections of a drug called Faslodex every month, with an extra dose halfway through the first month.
It is important to understand that these particular drugs for metastatic breast cancer are not traditional (cytotoxic) chemotherapy drugs. What I mean by that is that most people think about chemo as being drugs you receive, most often via IV, that makes you feel rotten and your hair fall out. That’s the type most people are familiar with and that class of drugs includes what I had when I had treatment for stage II breast cancer in 2007 (Adriamycin, Cytoxan (least creatively-named drug of all), and Taxol).
Those drugs are cytotoxic (cyto= cells, toxic= poisonous). So, the drugs kill the cancer cells but they kill other cells too. That’s why your hair falls out, you feel sick, your blood counts drop and a host of other issues.
With my kind of cancer (estrogen receptor positive, progesterone receptor positive, HER2 negative) there are other types of drugs to use to try to slow the cancer’s growth down. This is not the case for all types of breast cancer. With some you can only use traditional chemotherapy. In addition, and most importantly for this trial, my cancer shows a mutation in the Pi3k pathway. I know this is getting very science-y. But I’ll try to explain the rationale for this drug.
Many people with my kind of breast cancer (and other types of cancers, we’re learning) show a mutation in this pathway. You might have heard of genomic sequencing. It is testing the tissue of your cancer to see if your particular cancer has any mutations in its coding that facilitate the cancer’s growth (I’m oversimplifying here). This pathway, which is called the Pi3K/AKT/mTOR pathway, can become overactive and drive the cancer’s growth.
I have one mutation in the Pi3k section of the pathway. There are many forms of mutations in the Pi3k pathway. There can be other mutations in other areas as well. Mine is called a Pi3k-alpha mutation.
So what the investigational drug is targeted to do (hence the term “targeted therapy”) is to block this Pi3k/AKT/mTOR pathway that has been over-activated, potentially by this mutation.
This all sounds great. But it’s not so easy. It isn’t as easy as “find the mutation, create the drug, block the pathway, cancer goes away.” We don’t have indications it will ever be like that. The signaling pathways of cancer are highly complex, variable at any given point, and also change over time.
In addition, not everyone with the same mutation responds to the same drug. And combinations of drug seem to work better. Think about doing clinical trials of endless permutations of drug combinations with different mutations, different cancers, in different bodies… well, this is why science seems to move at lightning speed but our advances in treatment just don’t mirror that in all cancers. We have no way at the moment to predict what the best course of treatment is for any individual person. For now, you throw the pasta at the wall and see if it sticks. Unfortunately, our lives are the test cases.
To return to the science, I’ve already tried an mTOR inhibitor in my last treatment phase. That was called Afinitor (combined with another drug called Aromasin). But that targeted a different end of the pathway, and not the mutation. The problem with all of these right now is that the cancer figures out a way around the blockages. It develops feedback loops. If you’ll allow me to anthropomorphize cancer for a minute, it says, “Hey, okay, so you want to block the road? I’ll just detour and still get the end point. I’ll get fuel to the cancer somehow. If you block me, I’ll just keep finding a new way to deliver the goods.” And that’s why metastatic breast cancer is incurable. It keeps finding a way to find fuel and becomes resistant to each thing you throw at it. I became resistant to that Aromasin/Afinitor combination after about six months.
In terms of side effects, unlike traditional IV chemo, with this investigational drug you don’t “feel rotten” right after treatment. It can take weeks and potentially months for side effects (especially some of the serious ones) to take hold. So that’s one way this differs from what people might think. My phone has been buzzing non-stop since Monday: “How do you feel? How do you feel?” I will have some effects from the injections (which are hormonal agents, this one is an estrogen receptor agonist) quickly. I already have some of those. Other side effects from the pills (the GDC-0032) will come later.
So… here we go.
Monday was Cycle 1, Day 1.
If you are interested, my trial protocol is here (I am in the phase II group). This tells you exactly what this study is. If you want to read a bit about the early results of this drug in phase 1 trials you can see that here.
On day 1 I received my GDC-0032 pills for the month (and took the first dose), received two injections of Fulvestrant (Faslodex), and had about 8 vials of blood drawn.
The logistics of getting to the city were a bit of a challenge this week given fatigue and the lingering pleural effusion. The train, subway, and walk were tough but I always try to push myself. Knowing I’d be stuck inside for a few hours definitely had me enjoying the cool crisp fall air on the walk to the hospital.
Because of train times I arrived one hour early for my appointment. I wasn’t sure if I was doing this blood draw before seeing the doctor or after so I settled in. After only a few minutes I was surprised to be called back to an exam room. There I had a long meeting with research nurse whom I’ve spoken with by phone but not met in person. It was an extremely thorough meeting. She answered questions, reviewed the protocol, went through my current medications again, noted all physical symptoms I’m having now. We also discussed my most recent bloodwork (my lipids changed drastically during the 3 week washout period. My prior chemo had raised my cholesterol significantly. My LDL dropped a whopping 100 points in a two week period once I stopped the old chemo, for example). Blood pressure, pulse, oxygen saturation, height, weight. Other research assistants on the protocol come in and talk to me, discuss things, physical exam, as eventually does the Principal Investigator (the doctor in charge of the trial).
It took about two hours to complete these meetings, exams, tests, questions, medication review, etc. Everyone was very thorough and I was offered every opportunity to ask questions about not only the trial but also about any symptoms I was having and how they could be helped.
They also stressed how important it is to call with any and all side effects. As the more serious side effects become more possible/likely, it’s important to report any issues right away so they can be managed before they get too serious. Communication is key in clinical trials. I’ll talk about the side effects more in the future.
For some questions about side effects we needed to refer to the protocol of the trial (Can I get radiation to a bone if my bone pain continues: Yes, but not within the first two weeks of the trial start. Can I get the fluid around my lung tapped if it becomes too troublesome: Yes, at any time).
We also discussed a port for my blood draws/access for radioactive dye injections for CT and bone scans that I will need to have done every 8-12 weeks on the trial. I am still undecided about the port. We agreed to see how it goes in the next few weeks with the blood draws. I can only use my right hand which doesn’t allow for easy access or many misses. They tell me there is a “three miss rule.” If they can’t get the blood they need within three tries, they stop. I have a terrible feeling this rule will come in handy.
At the end of the meetings they handed me many sheets of paper.
First, I received a medication diary where I need to document the time I stop eating every night and the time I take my GDC-0032 pills (the “investigational drug”) the next morning. The pills must be taken on an empty stomach one hour before food, with a full glass of water at approximately the same time every day.
Second was a list of drugs and supplements I cannot take while I am on this protocol.
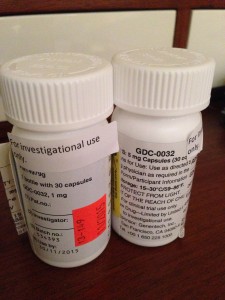
Finally, there was a schedule for the next 8 weeks of what will happen at each appointment, what tests and drugs I will receive, what I need to do to prepare, and what I need to bring to each appointment. The pills are given in quantities of 30 but I will probably be at appointments every 28 days. The surplus pills must be traded in and counted before I can receive the next month’s supply. Though it is only one drug it is dispensed in two capsules, one dark red, one pine green. One is a 5 mg, one is a 1 mg for a total of 6 mg.
I was then sent to do scheduling for my next appointments. I will need to return in two weeks (plus or minus 2 days) to do vital signs, exam, fasting blood draw (but this time it must be done twice: before taking my pills and then again 4 hours after), and my two injections.
I then went to the hospital pharmacy to collect my pills. They are labeled with my name, the drug name, instructions, and so on, just like a regular prescription. The protocol number is labeled on the bottles too. The bottles also say “Cost $0.00” on them.
This drug is provided free of charge to me but my insurance company is billed for the injections of Faslodex, lab work, and all scans. Trials differ in what is covered. In this case, only the investigational drug is provided free of charge. The rest of the expenses including the visits with the doctors are not provided for free. I am fortunate. My insurance will help pay for those things. Your care is not necessarily fully covered when you are in a trial, contrary to popular belief.
The pharmacy cashier placed the two bottles (one bottle of the 5 mg, one bottle of the 1 mg) in a brown paper lunch bag and handed it to me. In that moment I feel like I have something very valuable and secret in my possession, perhaps even magical. I don’t know if these pills will do anything for me. They could do me more harm than good. They could do nothing. But they might buy me time. Those are, for now, mysteries.
The brown paper bag with the drug bottles inside seems very wrong, far too flimsy for the weight of the hope that lies within.
I then left the pharmacy and headed upstairs to the chemo floor where I had my fasting bloodwork done (by now it was past 11 AM and I was glad I started early. I really don’t mind not eating, it’s the lack of coffee that gets me. Also, the longer I wait the harder the blood draw is likely to be).
In this room I will also receive my two injections. As I enter the room, I’m wondering about those and anxious. The number of needles I have in a month is astounding sometimes. I was the girl so afraid of shots as a kid I once ran into the parking lot to try to run away to avoid getting a vaccination at a pediatrician’s visit.
One chemo room is dedicated to this trial. I have gone to the same room each time so far. The trial tech doesn’t even try to draw my blood. He calls in the reinforcements. Eight or so vials of blood are taken by a nurse who goes after my hand vein with a vengeance. Unfortunately I receive a call the next day that two of the vials are unusable (hemolyzed). This happens sometimes when large blood draws require a lot of work (suction) to get. I was able to go locally the next day to have the two vials redrawn.
After the blood draw it was time for the “standard of care” drug. This is part two of my clinical trial protocol. The “standard of care drug” means I am given not just the experimental drug, but also a drug that is a reasonable option for treatment for this stage of my disease.
On its own (“single agent”) Faslodex works for some people but seems to have a better track record when combined with another agent. My trial is one that combines Faslodex injections (standard of care) with the GDC-0032 (the investigational drug). There is no placebo. This is a phase II trial. I will get both. We will see if taking the GDC-0032 provides a better result than the historical success rate of those who have received the Faslodex injections alone. There will be 60 people nationally doing this combo, about 10 of them at Sloan-Kettering. I am the fourth person, I believe, to get started on it (the trial just opened a few weeks ago at Sloan).
Faslodex is given by injection, intramuscularly, in your rear. No fancy fun way to say it. You drop your trousers and they have syringes that are over 4″ long (can’t find any literature that states exact measure. I’m going to ask to measure it after my shots next round. I confess I saw them when we were done and they made me a bit nauseated and I didn’t want to be precise!). The needles are thick because the liquid that has to be inserted is very viscous and doesn’t go into the muscle easily. I was fortunate and the nurse did a great job. I tried to remember the tip to keep your muscles relaxed during an intramuscular injection, but it’s hard when you know the size of the needle that’s taking aim for your ass.
The first injection really wasn’t worse than a regular shot. The second one hurt more than the first but still less than I had expected. I opened my bottles of pills, took out one of each, drank my water as directed. I noted the time in the diary.
At this point I was free to go. The injection sites were not immediately painful and I was sent on my way.
Everything was incredibly efficient and while I was exhausted, I was relieved. I kept thinking to myself: “Once again after three weeks of not being actively treating my cancer because of the mandatory ‘washout’ period, I am doing all I can. Action feels good.”
I celebrated getting through day 1 by having lunch with my friend Julie Klam which was such a luxury after the poking and prodding of the morning. I took the train home and felt a sense of accomplishment.
So, in case it wasn’t clear, my next appointment will be two weeks after my start date (“Cycle 1, day 15 +/- 3 days” in protocol speak.).
I think the word that most defines metastatic breast cancer to me is “uncertainty.” You have to figure out a way to live with it. My coping mechanisms are research and action. I can only hope these will serve me well.
I thank you for your support and encouragement this week.
September 30th, 2013 §
 Here we are again. A crossroads.
Here we are again. A crossroads.
The third of many we will have.
I’ll give a bit of a recap. I know it gets quite technical but documenting the details is important for those who also have metastatic breast cancer.
It’s almost one year to the day that I was diagnosed with metastatic breast cancer in my bones and lymph nodes. I started on the cytotoxic daily drug Xeloda first (in addition to monthly infusions of Zometa) which gave me six months of success, shrinking the amount of cancer evident on PET scans.
In April 2013 when my tumor markers started rising I started on the hormonal combo of Aromasin and Afinitor (and also switched from Zometa to Xgeva because of difficult side effects I was having). I started on a 5 mg/day dose of Afinitor with a 25 mg dose of the Aromasin. I gradually increased the Afinitor dose to 7.5 mg/day, and then when my markers began to rise again a few weeks ago, I started on 10 mg a day.
It has now been five months on this combo (in all of its different dose strengths) and my markers are rising. This chemo is no longer working. So, it’s time to decide what to do next.
Regular readers will know that this is typical of metastatic breast cancer: some therapies may not work at all while some will work for a while. Ultimately, though, the cancer becomes resistant to each of these treatments and will progress. Sometimes you get a great response and can reduce the amount of cancer that is present. Oftentimes you settle for the success of “stability” where the cancer isn’t reduced, but it also isn’t growing. It’s often referred to as hanging out with “Stable Mable.” This is still a good response in most people’s books.
Especially when your cancer is still confined to your bones and lymph nodes, stability has the added feature of being life-saving: as long as the cancer is in those places it cannot kill you. Only once it metastasizes to organs can breast cancer kill.
So… now it’s time to decide what to try next (this part gets technical). Right now it’s looking like I’m headed to a clinical trial of a drug that has shown promise in breaking the resistance of hormone sensitive breast cancers especially in people like me whose cancer displays a Pi3k mutation (40% of people with ER+ breast cancer have one of these mutations). This experimental drug would be in combination with a drug commonly used for my kind of cancer (Faslodex). I’ve enrolled with the clinical trial team. This is a Phase II trial so I would be guaranteed to get the experimental drug.
What does this mean? In simple English, it means I’m trying a new drug that’s designed to break the resistance of this particular kind of cancer to treatment. It seems to be especially effective for those with the type of cancer mutation that I have (Pi3k-alpha). If this drug makes the cancer susceptible to therapy, then the other drug I’m taking can do its work. This class of drugs is at the forefront of where cancer research trials are right now.
I’ve been pre-screeened and have a few more hurdles to clear: scans, bloodwork, and a three week “washout” period where I must be chemo-free to allow any residual effects of my current treatment to clear. Am I nervous about a period with no safety net? Yes, a bit. But I also am curious how my body will react to having current chemo clear my system. I’ve had a lot of trouble with things like high blood pressure, high cholesterol, rapid heart rate, shortness of breath, and headaches in the past few months. I’m interested to see how many of these will improve.
The new drugs will have their own series of side effects, some of them might be similar and a few may be different and more serious. So this three week period sits okay with me because if my current treatment isn’t doing anything anymore, it’s of no use anyway.
There will be so much to share about being involved in a clinical trial. And I will share that with you, should I make it through the final screening process. I am learning so much as I go and I think there is a real chance here to inform others about the experience. Trials are as varied as can be. Each has criteria that must be met. Sometimes you end up on the good side of these and sometimes you don’t.
My oncologist looked at a host of trials before we chose this one. I was ineligible for many of them because of the drugs I have already tried, my particular features of my cancer, what cities the trials were offered in, when they were enrolling patients, etc. Some were not attractive to me because of the phase of trial that it was (I’ll explain in a separate post the differences between Phase 1, 2 and 3 trials). I am glad to be in a trial where a drug has already passed initial safety standards and will also be given to every patient in the group (there are no placebos in this phase of trial).
It’s important to note that a clinical trial should not necessarily be viewed as a “Hail Mary” meaning it’s the last remaining hope. In reality, my sense is that this is very rarely the case, at least with Phase 2 or 3 trials. They can only establish true efficacy in people who are still at certain phases of the disease process and haven’t had too many chemotherapy agents already.
To me the key was to find a trial that had a good scientific basis for success, was currently enrolling patients and was within commuting distance. In addition, I think it’s best if the standard of care drug is one you would already be considering using at this point in your treatment. There is a database at www.clinicaltrials.gov where you can search for trials.
I have reserved my spot in this trial. If I meet the testing criteria I will be one of 60 patients nationwide on this particular protocol, about 10 at my particular location. I view it as an opportunity. It is well-suited for my cancer at this time in treatment. It’s using the newest thinking in targeted therapy based on genomic analysis.
It’s scary, yes. But if it doesn’t work I still have standby treatments to go to (and other trials by that time that I hopefully would be eligible for). I know there is no cure. But maybe this one will give me a chunk of time.
I won’t go into details here but I know people will ask what’s involved. The treatments will be injections (Faslodex is two big intramuscular injections in the butt every month after doses every 2 weeks the first month) combined with daily oral capsule (the experimental drug). I will also continue to get an Xgeva injection in the arm each month. There will be a strict schedule of fasting bloodwork in NYC every two weeks and scans every 8 weeks to monitor the cancer’s growth. I’ll have to keep a medication diary. I’ll let you know how everything else works once I am underway. It’s a lot of work, especially in the first two months including all of the meetings and tests just to get started.
There are so many questions I know people will have. If you post your questions in the comments I will do my best to answer them in future posts if I can. You may post them anonymously if you like. For now I’m not going to state the name of the drug (actually just a string of letters and numbers) until I’m actually enrolled.
It was quite devastating to get the news that the current chemo isn’t working anymore. It always feels like the rug being pulled out from under me. I always cry. I always feel like I’m falling.
But plans are my safety net. Options are my lifejackets.
I leap from treatment to treatment on my tippy toes, knowing if I place too much weight I will sink.
This is the dance I do now. Forever.
 Once again, it’s been a while since I’ve updated. Somehow most of you know that with me usually no news does not actually equal good news. Before I give an update I want to remind readers that I do update the blog Facebook page (click here to reach it) with short medical updates more often these days if you are interested.
Once again, it’s been a while since I’ve updated. Somehow most of you know that with me usually no news does not actually equal good news. Before I give an update I want to remind readers that I do update the blog Facebook page (click here to reach it) with short medical updates more often these days if you are interested.





 Link to Twitter
Link to Twitter